
VIVA Cell and Gene Therapy Symposium 2025
21 June 2025 | 9:00AM - 5:30PM (GMT+8 SG Time)*
The VIVA Cell and Gene Therapy Symposium, proudly sponsored by Miltenyi Biotec, returns for its third edition as a premier gathering dedicated to advancing the frontiers of clinical cellular immunotherapy. Building upon the success of previous years, this symposium continues to serve as a pivotal platform for healthcare professionals to converge and explore the latest advancements and breakthroughs in the dynamic field of cell and gene therapies.
With the potential to revolutionise medical treatments and address previously incurable diseases, cell and gene therapies have garnered significant attention and excitement. This symposium remains steadfast in its commitment to fostering collaboration, accelerating scientific progress, and catalysing the translation of cutting-edge discoveries into tangible clinical applications.
The full-day program will feature expert-led lectures and panel discussions, providing insights into cutting-edge research and clinical applications. In-person attendees will also have the opportunity to participate in a complimentary hands-on workshop focusing on CAR T cell manufacturing, offering practical experience with state-of-the-art technologies.
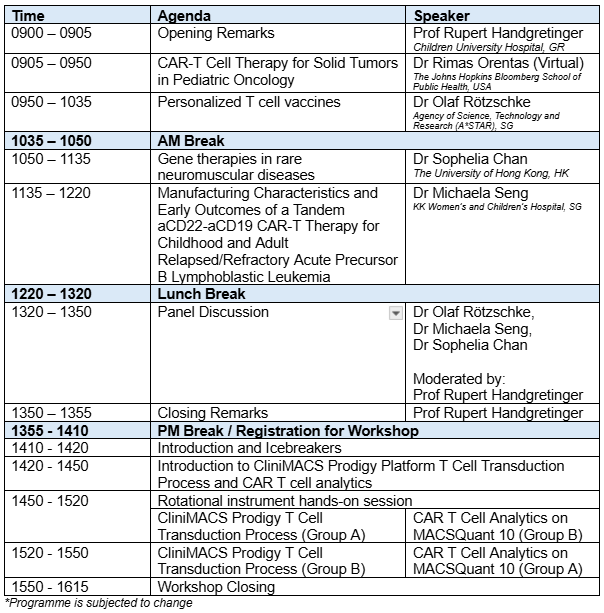
PROGRAMME RUNDOWN
WATCH IT AGAIN
PRESENTATION SUMMARIES
CAR-T Cell Therapy for Solid Tumors in Pediatric Oncology by Dr Rimas Orentas
The rapid advances in chimeric antigen receptor (CAR) engineered T cells as a treatment modality for hematologic oncology for pediatric diseases has not been matched with similar advances in either central nervous system arising- or peripheral malignancy. While direct intravenous administration of CAR-T cells for solid tumors and even advanced delivery methods for CNS tumors, such as intracranial administration of the therapeutic cell product, have at times shown transient responses, we are still wanting for a major breakthrough. To optimize the CAR T cell approaches we have engineered T cells to express FGFR4-targeted CARs for rhabdomyosarcoma and IL1-RAP specific CARs for Ewing sarcoma. In both cases promising pre-clinical studies have highlighted the unique challenges posed by the tumor microenvironment. Uniquely evolved to avoid effective immunosurveillance, the tumor microenvironment and additional physiological challenges posed by systemic therapy of solid tumors, demands new strategies to armor T cells and to further engineer their biology to be devised. Our goal is to provide durable cures to children with cancer when CAR-T cell therapy is applied to the challenge of relapsed and chemo-refractory disease.
Gene therapies in rare neuromuscular diseases by Dr Sophelia Hoi Shan Chan
Recent advancements in technology have led to the development of new treatments for neuromuscular diseases that were previously managed only with supportive care. Gene therapies for conditions such as spinal muscular atrophy, Duchenne muscular dystrophy, limb-girdle muscular dystrophy, myotubular myopathy, and hereditary motor and sensory neuropathy type 1A are currently being evaluated in clinical trials, showing promising early results. The FDA and EMA have approved onasemnogene abeparvovec for spinal muscular atrophy, and the FDA has approved delandistrogene moxeparvovec-rokl. This review explains gene therapy principles, summarizes current results, and provides an overview of ongoing and upcoming clinical trials. While gene therapies could significantly impact these diseases, long-term effects and safety data are still needed. These new treatments will also bring challenges for healthcare systems regarding diagnosis, treatment, and cost.
WORKSHOP DETAILS
Chimeric Antigen Receptor (CAR) T cell therapy has revolutionized cancer treatment by enabling patient-derived T cells to target malignant cells. Manufacturing involves T cell activation, genetic modification, expansion, and quality control. Despite its success, challenges like long production times, high costs, and variability remain. Advances in automation and closed-system bioprocessing aim to improve efficiency and scalability.
The Miltenyi Biotec' CliniMACS Prodigy Platform streamlines the entire process within a single device. By reducing manual interventions and contamination risks, the CliniMACS Prodigy enhances process reproducibility and efficiency, making CAR T cell therapy more accessible. Complementing the CliniMACS Prodigy in CAR T cell manufacturing is the MACSQuant Analyzer 10. As a powerful flow cytometry platform designed for automated, high-throughput, and multiparametric analysis of CAR T cell products, it enables precise characterization of key quality attributes, including transduction efficiency, phenotypic composition, viability, and purity, ensuring compliance with regulatory standards.
This workshop explores the impact of the CliniMACS Prodigy and MACSQuant Analyzer 10 on CAR T cell manufacturing, its advantages over traditional methods and potential to enhance the scalability and reliability of cell therapy production. A certificate of participation will be issued upon completion of the workshop.
MODERATOR/ ORGANSINING CHAIR

Prof Rupert Handgretinger
Professor Emeritus
Children's University Hospital, Tübingen, Germany
Consultant, Abu Dhabi Stem Cell Center and Yas Clinic Khalifa City, Abu Dhabi, UAE
George and Jennifer Yeo Endowed Chair in Pediatric Oncology, Yong Loo Lin School of Medicine,
National University of Singapore
Singapore
SPEAKERS

Dr Rimas Orentas
Sr. Scientific Director, Immunotherapy, Miltenyi Biotec
Professor (Adj.), The Johns Hopkins Bloomberg School of Public Health, Biochemistry and Molecular Biology
USA

Dr Sophelia Hoi Shan Chan
Clinical Associate Professor, Dept. of Paediatrics and Adolescent Medicine, School of Clinical Medicine, The University of Hong Kong
Neurology Team Lead, Hon. Consultant, Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital
Honorary Consultant, Department of Pediatrics and Adolescent Medicine, Queen Mary Hospital, Duchess of Kent Children’s Hospital, The Hong Kong University -Shenzhen Hospital,
Hong Kong

Dr Olaf Rötzschke
Senior Principal Investigator,
Singapore Immunology Network (SIgN),
Agency of Science, Technology and Research (A*STAR)
Singapore

Dr Michaela Seng
Senior Consultant, Children’s Blood and Cancer Centre
Dept of Paediatric Haematology and Oncology
KK Women’s and Children’s Hospital
Duke-NUS Medical School
Singapore




