VIVA Cell and Gene Therapy Webinar #1
27 September 2024, Fri | 8:00PM - 9:00PM (GMT+8 SG Time)*
Join us for an insightful webinar on the Cell Manufacturing Enabling Package, a groundbreaking approach to streamline point of care CAR T cell manufacturing. Our expert speakers, Dr. Hoàng Thanh Vân and Dr. Nguyễn Thanh Liêm from the Vinmec Research Institute of Stem and Gene Technology in Vietnam, and Dr. Saskia Röesch, Global Product Manager T Cell Immunology at Miltenyi Biotec B.V. & Co in Germany, will delve into the latest advancements and share their experiences in harnessing the potential of cell and gene therapy. This webinar will take place on 27th September from 8pm to 9pm. Secure your spot now and be part of this exciting discussion!
PRESENTATION SUMMARY
CAR T cell therapy process development and validation support in PoC setting by Dr Saskia Roesch
For clinicians who want to start manufacturing CAR T cells, setting up a cell manufacturing process and QC system that can be transferred to GMP-compliant standards is extremely challenging. The development of a viable protocol is time-consuming and expensive, and translating protocols into SOPs is complicated, often resulting in further delays and expenses. Learn how the Cell Manufacturing Enabling Package (CMEP) can save you time on process development and validation. Designed by experts, and tested through years of experience in the CliniMACS Prodigy® cell manufacturing facility, it has been developed to accelerate your path toward clinical manufacturing.
Developing the first point-of-care manufactured CAR T-Cell Therapy in Vietnam by Dr Van T. Hoang and Prof Nguyen Thanh Liem
CAR T-cell therapy shows promise as a treatment for acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). Vinmec initiated CAR T-cell research in 2016, but encountered various laboratory and clinical challenges. In 2019, we sought global collaboration to conduct Vietnam’s first CAR-T clinical trial. Miltenyi has provided crucial support during this journey. Between December 2022 and March 2023, Miltenyi experts trained Vinmec scientists in CAR T-cell manufacturing using Prodigy and quality control (QC) on MACS Quant 10 (known as the CMEP training package).
Since August 2023, Vinmec has conducted a phase 1 clinical trial to evaluate the safety and efficacy of point-of-care-manufactured CD19 CAR-T cell therapy. To date, eight ALL and four NHL patients have been treated with CD19 CAR T-cells. Common side effects included cytokine release syndrome (in 10 patients) and neurological toxicity (in 2 patients). CAR T-cells expanded in vivo, peaking between days 10 and 14, coinciding with blast clearance in peripheral blood and bone marrow. Follow-up duration ranged between 1 and 10 months. 2 of 3 NHL patients (with one patient awaiting evaluation) achieved complete remission after CAR T-cell infusion. 7 of 8 ALL patients achieved complete remission, while one ALL patient was refractory and died after 30 days. During follow-up, two NHL and four ALL patients remained in complete remission, one NHL and three ALL patients experienced relapse. In conclusion, point-of-care CAR T-cell manufacture is feasible in developing countries like Vietnam. Our initial results demonstrated that CAR T treatment is relatively safe and holds promise for refractory/relapsed CD19+ NHL and ALL patients.
WATCH IT AGAIN!
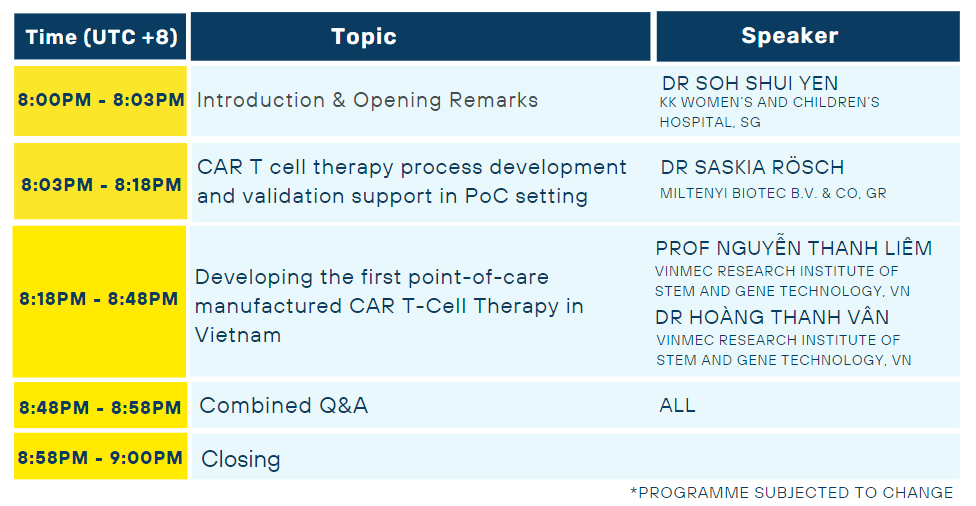
PROGRAMME RUNDOWN
SPEAKERS

Prof Nguyen Thanh Liem
Director of Vinmec Research Institute of Stem and Gene Technology,
Director of Department of Regenerative Medicine & Cell Therapy, Vinmec International General Hospital,
Director of Vietnam Association of Regenerative Medicine & Cell Therapy
Honorary lecturer at Colleague of Health Science, Vin University
Vietnam

Dr Saskia Rösch
Global Product Managers T Cell Immunology
Miltenyi Biotec B.V. & Co
Germany

Dr Hoàng Thanh Vân
Head of Department
Department of Applied Clinical Research
Vinmec Research Institute of Stem Cell and Gene Technology
Vietnam
MODERATOR

Dr Soh Shui Yen
Head & Senior Consultant Haematology/Oncology Service
Paediatric Brain and Solid Tumour Programme
KK Women's and Children's Hospital
Singapore